
A FIRE is a chemical reduction of complex organic substances into simpler compounds.
Basically, it is the combination of combustible material and oxygen, with a resultant emission of heat, flame, and vapours.
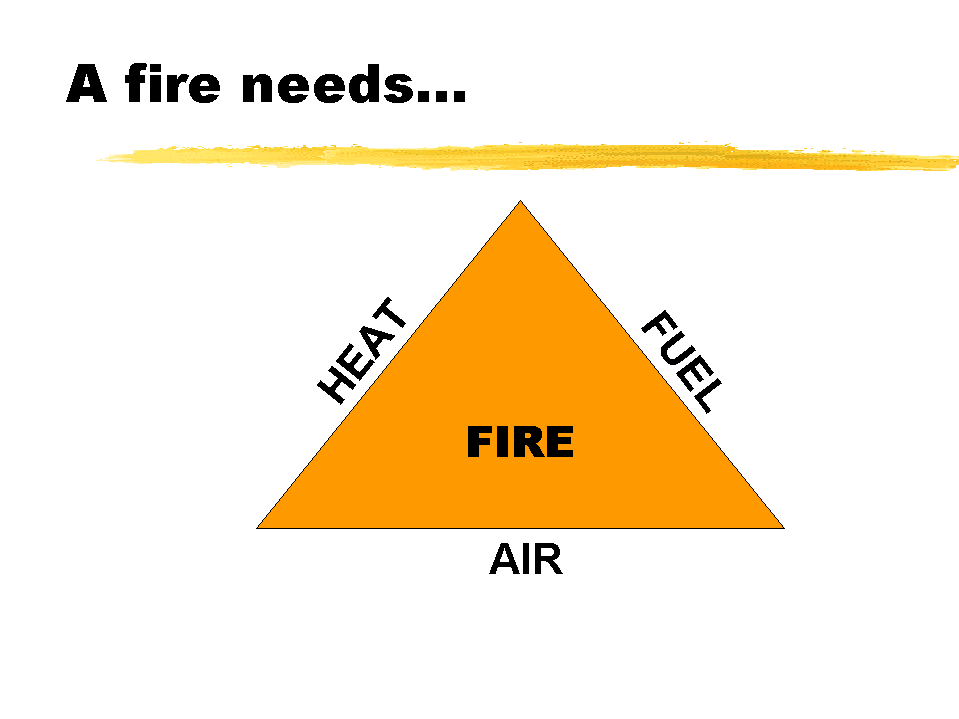
For a fire to exist, the three elements represented on each side of the triangle must be available.
AIR: normal air contains around 21% oxygen, so is an important ingredient in feeding a fire.
FUEL: is generally available in the form of a flammable vapour. Carbon and some metals are exceptions to this rule.
HEAT: in sufficient quantity allows the combustible material to emit its flammable vapours, ignite and combine with oxygen. In timber fires, the heat released is the solar energy which plants have converted though the process of photosynthesis.
REMEMBER: IF YOU REMOVE ANY SIDE OF THE TRIANGLE YOU WILL EXTINGUISH THE FIRE.
Starve the fire of fuel: eg remove combustible material, shut off gas valves, provide a barrier from the fire.
Starve the fire of air: eg cover the combustible material with a fire blanket, displace oxygen-rich air with carbon dioxide.
Cool the materials to prevent flammable vapours from being released: eg water, foam, CO2, etc. - however: use only the correct extinguishers on electrical and/or oil based fires.
In the event of fire, the R.A.C.E. rule can be followed (in any order):
· Remove non-essential personnel;
· Alert appropriate authority;
· Contain and isolate the fire if you are able, so as to minimise its spread;
· Extinguish it if you can do so with safety.
Know your limitations!
Fire extinguishers are basically to give you time to evacuate an area. Smoke and fumes in modern fires contain combinations of dangerous substances, and are likely to cause more casualties than heat and flame. Do not linger!
Several memory triggers for fire extinguisher identification are:
CO2 (Carbon dioxide): What colour is carbon?
BLACK!
So when we see a red extinguisher with a BLACK band, think CARBON dioxide. Good for most fires, except gas fires. Caution: displaces oxygen in confined spaces.
Dry chemical powder: What colour is baby powder?
WHITE!
When we see a red fire extinguisher with a WHITE band, think POWDER. Good for most fires. Makes a bit of a mess, but hey! Saving lives is the prime aim!
Water: What colour is water?
CLEAR!
When we see all-red fire extinguisher with no band, think of it as having an all-CLEAR band. Good for wood, paper, solid plastics, etc. Do not use on electrical fires or liquid petroleum or alcohol based fires.
Foam: The memory trigger here is a little more obtuse - What floats around on top of the deep BLUE sea?
FOAM!
When we see an all-BLUE fire extinguisher, remember the ocean, and FOAM. Good for wood, paper, plastics, etc., as well as most liquid petroleum fires. Caution: conducts electricity.
Wet chemical: Think of 'Joe's Greasy Spoon Restaurant' - officially, OATMEAL is the colour, but think of BROWN.
This all-Brown extinguisher is good for paper, etc., but is particularly the extingusher we should find around deep frying fats and cooking oils in kitchens, etc., where the chemical foam is particularly efective. Caution: conducts electricity.
Halon - BCF vapourising liquid: Think of the Sun, think of YELLOW.
This all-YELLOW extinguisher is generally banned due to halon's destructive powers on the ozone layer, which then allows the Sun's ultra-violet rays through to harm life. However, some hi-tech facilities are given permission to retain them due to their efficiency around electrical fires. Inadvisable to be present when they are used, due to dangerous fumes emitted.
Welcome Page | About Us | Services | Pricing | Book of Triangles | Contact Us | Other Safety Sites